Imagine a future hospital where specialized treatment prompts a patient's damaged thyroid to regenerate into a healthy organ. Or where a lost finger can grow back.
That sort of recuperative magic is impossible today, and maybe it never will happen. But if it does take place, there's a good chance that the line of discoveries leading to such medical miracles will include a find by a team of University of Utah scientists.
Their discovery, concerning the way a species of small primitive animals called flatworms can regenerate, was announced in Friday's edition of the journal Science. The team was led by Alejandro Sanchez Alvarado, an investigator at the Howard Hughes Medical Institute at the U. and professor in the U.'s School of Medicine.
They found that a protein produced by a gene called smedwi-2 seems to be required to produce adult stem cells that, in flatworms, are capable of replacing aged cells and replacing missing tissue during regeneration, says a summary by Science.
A stem cell is a basic, undifferentiated cell that can produce offspring cells of many different types, such as blood cells or kidney cells. Today, stem cell research is one of the most important areas of science.
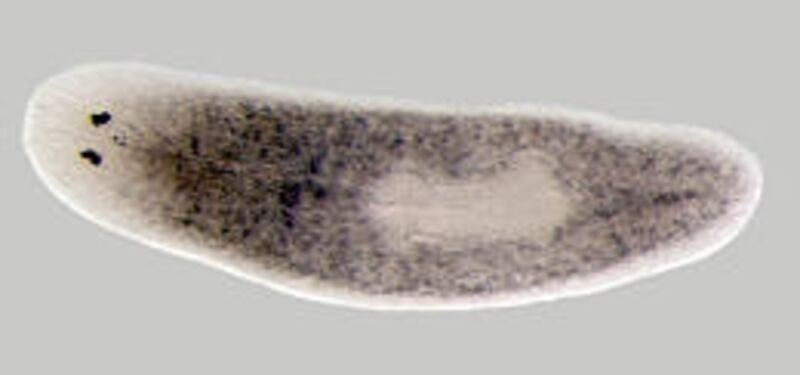
Sanchez Alvarado said the scientists decided to study the freshwater worms "because they have a large amount of stem cells in their bodies, and they are renowned for their regenerative ability.
"These animals can be sliced and diced in a variety of ways, and these fragments will regenerate." If a flatworm is cut into three pieces, each piece will grow into a complete flatworm.
Genes flatworms use to regenerate are shared by many forms of life, including plants, nematodes and humans. The genes are "associated in the regulation of stem cells in organisms as different as plants and humans," he said.
A goal of the research was to discover the biological function of smedwi-2. When the team used radiation to knock out the gene in flatworms, they found that the stem cells remained.
"What's really affected was the capacity of the division progeny, the 'daughters' of these stem cells, to turn into differentiated cells," he said.
When the targeted gene was damaged, the molecular instructions that their daughter cells need to perform their healing work apparently were missing.
"It was really a surprise to us," Sanchez Alvarado said.
Earlier studies had suggested that the gene's function was the maintenance of stem cells, but they found that instead, it involved the ability of stem cells to turn into the particular types of cells needed in repair and regeneration.
With the gene gone, "the daughter cells of these stem cells cannot replace tissue that's dying due to age or missing parts of the animal after amputation. So they basically lose the entire regenerative capacity."
In the human genome, the gene has some involvement with blood. "But it's actual function is not clear," he said. "It's not known yet."
The next step is to discover if the gene has a similar function in higher organisms. "That work will have to be confirmed by other laboratories," Sanchez Alvarado said.
What steps even higher on the stairway — the future regeneration of human organs?
The line of research his crew was following was aimed at understanding how animals can regenerate, he said.
"It's a little bit too early to project the applications of these findings to human therapy," Sanchez Alvarado added.
Other members of his lab worked on the project, including Peter W. Reddien, now with the biology department at the Massachusetts Institute of Technology, Cambridge. Other researchers are Nestor J. Oviedo, now at Harvard Medical School, and Joya R. Jennings and James C. Jenkins of the Howard Hughes Medical Institute.
E-mail: bau@desnews.com