The Food and Drug Administration granted a promising Alzheimer’s treatment fast-track approval on Friday, the agency said in an announcement.
Early clinical trials reported that lecanemab, an experimental drug created by Eisai and Biogen, slowed the progression of the disease, as Lois M. Collins reported for the Deseret News.
What is Alzheimer’s? How can lecanemab help?
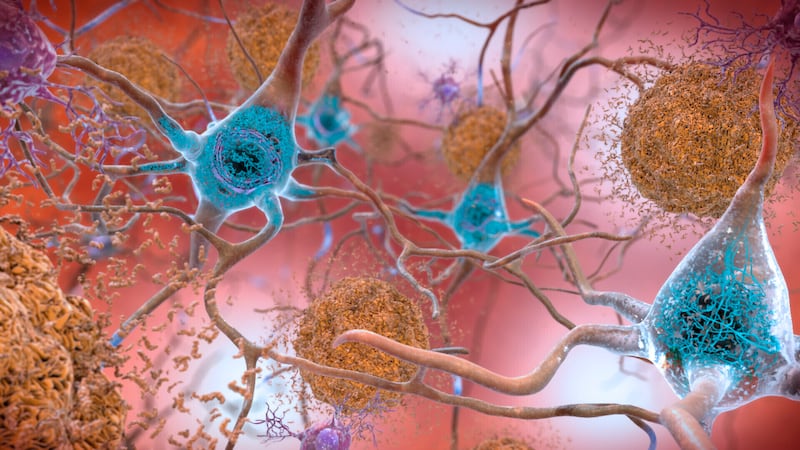
The disease is an irreversible and progressive brain disorder that affects memory and thinking skills. According to the Alzheimer’s Association, more than 6 million Americans suffer from Alzheimer’s.
“Alzheimer’s therapies will only be beneficial to patients if the right drug is given to the right patient at the right time based on their unique disease pathology, and for that we need new and novel diagnostic biomarkers,” Howard Fillit, co-founder and chief science officer of the Alzheimer’s Drug Discovery Foundation, told Axios.
In the trials, the drug reduced the presence of amyloid beta plaque, a marker of Alzheimer’s.
“This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer’s, instead of only treating the symptoms of the disease,” said Dr. Billy Dunn, director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, per the release.
Does the new treatment have side effects?
As Collins reported in November, lecanemab, which will be marketed as Leqembi, was associated with severe side effects. About 1 out of 8 people who used the treatment experienced brain swelling, while 1 in 7 experienced bleeding in the brain. Two patients involved in the trial died.
These will be mentioned on a warning label, alongside a list of other side effects, the FDA said. Eisai in a press release said lecanemab will cost $26,500 per year.